ULTIFEND™ IBD ND + SB-1 contains a genetically engineered Marek’s disease vaccine of serotype 3 (turkey herpesvirus or HVT) expressing infectious bursal disease and Newcastle disease key protective antigens, and a serotype 2 (SB-1) Marek’s disease vaccine. This bivalent Marek’s disease vaccine containing serotypes 2 and 3 is presented in a frozen cell associated form in 2 ampoules. One ampoule contains the HVT IBD ND fraction and one ampoule contains the SB-1 fraction. The cells and virus particles are very fragile and require careful handling to prevent damage or loss of titer in order to achieve optimum efficacy. The vaccine is stored and shipped in frozen form in liquid nitrogen.
ONE HATCHERY APPLICATION TO HELP OPTIMIZE YOUR GUMBORO, NEWCASTLE AND MAREK’S PROTECTION AND BIRD PERFORMANCE.
Your Ultimate Solution for IBD & ND
Ultifend is a vaccine consisting of an HVT virus which expresses the VP2 gene of the Infectious Bursal Disease virus (IBD) and the F gene of the Newcastle Disease virus (ND).
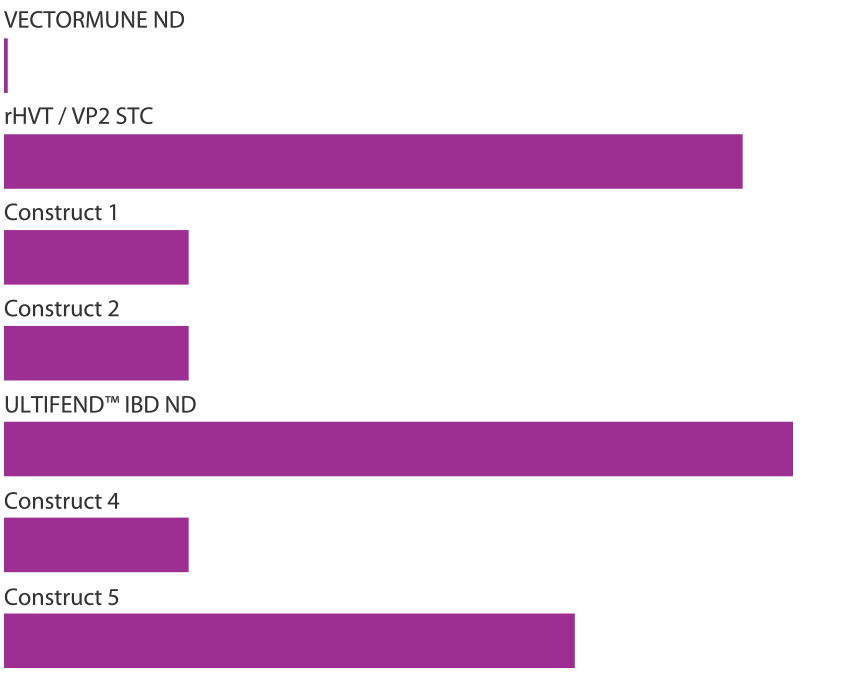
ULTIFEND™ IBD ND was selected from among 40 different vaccine candidates as the best construct for optimal protection against IBD, ND and Marek’s disease. Construct 3 was selected based on similar ND protection as VECTORMUNE® ND and similar IBD protection as rHVT/VP2 STC.
Now Available with SB-1
IBD Protection
ND Protection
IBD Protection
ULTIFEND™ IBD ND at minimum protective dose achieved the 9 CFR requirement of at least 90% protection from standard IBD lesions.
Eighteen days old embryos were vaccinated via in ovo or day of age chickens were vaccinated via subcutaneous and challenged at 5 weeks of age with IBDV-STC challenge strain via eye-drop, according to 9 CFR. Necropsy to score for bursa lesions were performed to evaluate the percent protection of the vaccinates in comparison to non-placebo-vaccinated chickens (Study on file with CVB).
ND Protection
ULTIFEND™ IBD ND at minimum protective dose achieved the 9 CFR requirement of at least 90% protection from mortality induced by ND virus infection.
Eighteen days old embryos were vaccinated via in ovo or day of age chickens were vaccinated via subcutaneous and challenged at 4 weeks of age with Texas GB via IM injection according to 9 CFR (Study of file with CVB).
Marek’s Disease Protection
ULTIFEND™ IBD ND at minimum protective dose achieved the 9 CFR requirement of at least 80% protection from tumor lesions caused by virulent GA Marek’s Disease virus.
Eighteen days old embryos were vaccinated via in ovo or day of age chickens were vaccinated via subcutaneous and challenged at 5 days of age with virulent Marek’s disease virus GA strain according to 9 CFR (Study on file with CVB)
Marketed by: Ceva Animal Health, LLC | Tel: 913-894-0230 | 8735 Rosehill Road, Ste. 300
Lenexa, KS 66215 | Ultifend trademark is the property of Ceva Santé Animale S.A.